Hydrocephalus Shunt System
Price 40-50 USD ($)/ Piece
MOQ : 500 Pieces
Hydrocephalus Shunt System Specification
- Instruments Type
- Implantable Shunt
- Diameter
- Tubing: 1.2mm - 3mm
- Surface Finish
- Smooth
- Disposable Or Reusable
- Disposable
- Finish Type
- Smooth Polished
- Function
- Diverts excess cerebrospinal fluid from brain to peritoneal cavity
- Instrument
- Hydrocephalus Shunt System
- Type
- Ventriculoperitoneal Shunt
- Material
- Medical Grade Silicone and Plastic
- Condition
- New
- Technology
- Pressure-controlled valve system
- Portable
- Yes
- Rechargeable
- No
- Foldable
- No
- Sterilized
- Yes (Pre-sterilized, ready for use)
- Waterproof
- Yes
- Use Type
- Neurosurgical
- Usage
- Hydrocephalus fluid diversion
- Operating Type
- Manual
- Dimension (L*W*H)
- Varied sizes available, typically 30cm length
- Weight
- Approx. 30 grams
- Application
- Management of Hydrocephalus (CSF drainage)
- Features
- Anti-siphon, MRI compatible, kink-resistant tubing
- Type Of Equipment
- Neurosurgical Implant
- Size
- Pediatric and Adult sizes available
Hydrocephalus Shunt System Trade Information
- Minimum Order Quantity
- 500 Pieces
- FOB Port
- MUMBAI
- Payment Terms
- Western Union, Paypal, Cash Advance (CA), Telegraphic Transfer (T/T), Cash in Advance (CID)
- Supply Ability
- 1000 Pieces Per Week
- Delivery Time
- 1-2 Week
- Sample Available
- Yes
- Sample Policy
- If order is confirmed we will reimburse the sample cost
- Packaging Details
- Poly Pack
- Main Export Market(s)
- Australia, North America, South America, Eastern Europe, Western Europe, Middle East, Africa, Central America, Asia
- Main Domestic Market
- All India
- Certifications
- ISO,CE
About Hydrocephalus Shunt System
Hydrocephalus Shunt System contains following parts as per requirement:
- Valve, Valve with Reservior, Anti-Gravity Valve
- Distal Catheter, Ventricular Catheter
- Connector, Y-Connector, Right Angle Connector
- Cerebral Catheter Reservior
- Ventricular Catheter Passer
- Subcutaneous Catheter Passer
- Material Used: Medical Grade Polymer
- Packing: Poly Pack
Reliable Hydrocephalus Management
This shunt system employs a pressure-controlled valve for precise regulation of CSF drainage, reducing risks associated with over- or under-drainage. Its radiopaque strip enables easy monitoring through imaging techniques, and smooth, kink-resistant tubing maintains consistent fluid flow.
Versatile Application Across Age Groups
Designed to accommodate neonates, pediatric, and adult patients, the system is available in a range of sizes. It is a versatile solution for neurosurgeons managing varying degrees of hydrocephalus in both acute and long-term situations.
Enhanced Safety and Convenience
MRI compatibility up to 3 Tesla and latex-free composition ensure patient safety. The disposable, sterile, and waterproof design allows for straightforward surgical use with minimal risk of infection or complication.
FAQ's of Hydrocephalus Shunt System:
Q: How does the pressure-regulated valve in this hydrocephalus shunt system function?
A: The pressure-regulated (fixed-pressure) valve ensures that cerebrospinal fluid is diverted at a controlled rate, typically within the range of 20 to 50 mL/hr, depending on the model. This precise control helps maintain optimal intracranial pressure and reduces risks of over- or under-drainage.Q: What patients are best suited for this shunt system?
A: This shunt is suitable for neonates, pediatric, and adult patients diagnosed with hydrocephalus who need controlled CSF diversion. Multiple sizes are available, allowing physicians to tailor treatment for individual needs.Q: When should the hydrocephalus shunt system be used?
A: The system is intended for use during neurosurgical procedures aimed at managing hydrocephalus by diverting excess CSF from the brain to the peritoneal cavity. It is used both in initial treatments and in revisions or replacements when necessary.Q: Where can this shunt system be utilized?
A: Designed for hospital and neurosurgical operating rooms, the shunt is supplied sterile and individually packed for immediate use by trained healthcare professionals in sterile environments.Q: What is the process for installing the hydrocephalus shunt system?
A: A neurosurgeon implants the shunt system using a sterile technique, connecting the silicone tubing between the ventricular space in the brain and the peritoneal cavity. The standard Luer lock connectors ensure secure attachment, and the system's radiopaque strip assists with imaging verification during and after placement.Q: What are the main benefits of using this shunt system?
A: Key benefits include controlled pressure regulation, MRI safety up to 3 Tesla, clear radiographic visualization, latex-free construction to reduce allergic reactions, and kink-resistant, anti-siphon design that minimizes complications.Q: How is the hydrocephalus shunt system maintained and is it reusable?
A: The system is single-use (disposable) and does not require post-implant maintenance. It is supplied pre-sterilized for immediate use, and replacement is only needed if warranted by clinical indications, such as shunt malfunction or infection.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Surgical Instrument Category
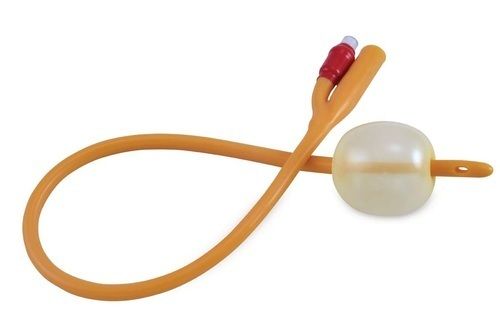
Foley Balloon Catheters
Price 0.50-1 USD ($) / Piece
Minimum Order Quantity : 5000 Pieces
Material : Other, Latex or Silicone
Condition : New
Portable : Yes
Disposable Or Reusable : Other, Disposable
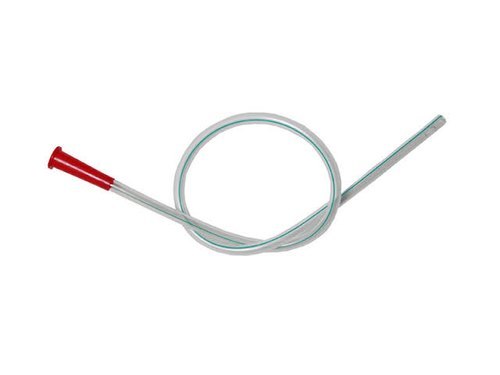
Levin s Tube
Price 20.0 USD ($) / Unit
Minimum Order Quantity : 100 Units
Material : Other, Medical Grade Polyvinyl Chloride (PVC)
Condition : New
Portable : Yes
Disposable Or Reusable : Other, Disposable
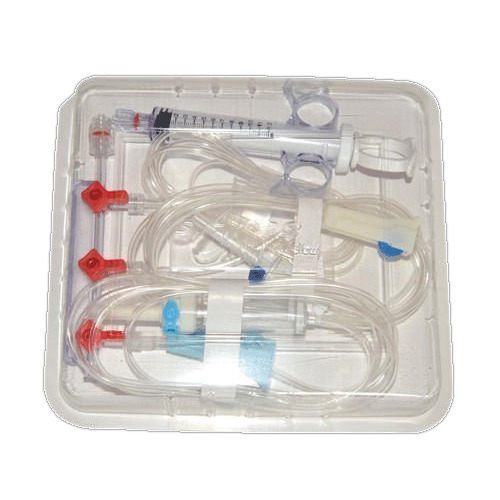
Angiography Kit
Price 8 INR / Kit
Minimum Order Quantity : 1000 Kits
Material : Plastic
Condition : New
Portable : Yes
Hand Control Pencil
Price 2 INR / Piece
Minimum Order Quantity : 2500 Pieces
Material : Plastic
Condition : New
Portable : Yes

 Send Inquiry
Send Inquiry Send Inquiry
Send Inquiry